Implant scandal sparks EU medical device
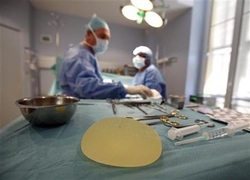
Implant scandal sparks EU medical device rule revampBy Charlie Dunmore and Kate Kelland | Reuters – Wed, Sep 26, 2012
BRUSSELS/LONDON (Reuters) - The European Union said it wants tougher rules governing the safety and monitoring of medical devices after weak EU regulations were partly blamed for a global scandal over French-made breast implants.
The plans will affect a huge range of products, from contact lenses to pacemakers to pregnancy testing kits to high-tech medical equipment such as life support machines.
Major manufacturers of medical devices include Johnson & Johnson, Medtronic, Boston Scientific,Abbott Laboratories, Allergan and Smith & Nephew.
Investigations last year showed that hundreds of thousands of women around the world had been implanted with substandard silicone products made by defunct French company Poly Implant Prothese (PIP), which safety regulators had failed to stop for more than a decade.
The scandal prompted calls for Europe to toughen controls on medical devices, which are currently overseen by an ad hoc network of up to 80 national assessment agencies.
"Everybody was shocked by the scandal involving fraudulent breast implants which affected tens of thousands of women in Europe and around the world," EU health commissioner John Dalli said as he outlined draft proposals for the new rules on Wednesday
The proposals take into account lessons from the PIP implant scandal AND include a scrutiny panel which would monitor the national agencies' assessments.
"They (the panel) would have the possibility to pick out medical devices on certain risk-based criteria to decide whether to go into an in-depth analysis of the processes," Dalli told reporters at a news conference.
INDUSTRY AND CONSUMER GROUPS CONCERNED
Industry body Eucomed, which represents about 22,500 medical technology companies in Europe, said it was unhappy with the proposal for the new scrutiny panel procedure, which it said would "hamper innovation" whilst providing no extra safety nets for patients.
"The current regulatory framework has provided a high level of safety for patients in Europe without delaying them access to life-saving medical technologies," Serge Bernasconi, Eucomed's chief executive, said in a statement.
"Let's not unnecessarily push away Europe's strong innovation and research capabilities to other continents at a time when they are urgently needed."
European Consumer Organisation (BEUC) argued, on the other hand, that the plans fall short of increasing quality and safety standards and said medical device regulations should be beefed up to levels similar to those required for pharmaceuticals.
"It is unacceptable that consumers are afforded different protection levels depending whether they have an artificial heart valve or take medicine for diabetes," said Monique Goyens, head of BEUC.
She noted that if a there is a problem with a drug, patients can stop taking them, but if an implanted device is problematic, patients may face invasive and risky surgery to have it removed.
Among other main changes proposed is an extension of the current legal definition of medical devices to include breast and other aesthetic implants.
Independent assessment agencies will be given greater powers to monitor device manufacturers, including unannounced factory inspections and regular product testing, while EU governments will be obliged to improve their supervision of the agencies.
Better product traceability systems will also be introduced so that people can be alerted more rapidly to safety concerns surrounding a particular device.
The European market for medical devices was estimated at 95 billion euros (123 billion) in 2009.
The legislation must be jointly approved by EU governments and lawmakers, which could take up to two years.
($1 = 0.7715 euros)
(Editing by James Jukwey and Hans-Juergen Peters)
BRUSSELS/LONDON (Reuters) - The European Union said it wants tougher rules governing the safety and monitoring of medical devices after weak EU regulations were partly blamed for a global scandal over French-made breast implants.
The plans will affect a huge range of products, from contact lenses to pacemakers to pregnancy testing kits to high-tech medical equipment such as life support machines.
Major manufacturers of medical devices include Johnson & Johnson, Medtronic, Boston Scientific,Abbott Laboratories, Allergan and Smith & Nephew.
Investigations last year showed that hundreds of thousands of women around the world had been implanted with substandard silicone products made by defunct French company Poly Implant Prothese (PIP), which safety regulators had failed to stop for more than a decade.
The scandal prompted calls for Europe to toughen controls on medical devices, which are currently overseen by an ad hoc network of up to 80 national assessment agencies.
"Everybody was shocked by the scandal involving fraudulent breast implants which affected tens of thousands of women in Europe and around the world," EU health commissioner John Dalli said as he outlined draft proposals for the new rules on Wednesday
The proposals take into account lessons from the PIP implant scandal AND include a scrutiny panel which would monitor the national agencies' assessments.
"They (the panel) would have the possibility to pick out medical devices on certain risk-based criteria to decide whether to go into an in-depth analysis of the processes," Dalli told reporters at a news conference.
INDUSTRY AND CONSUMER GROUPS CONCERNED
Industry body Eucomed, which represents about 22,500 medical technology companies in Europe, said it was unhappy with the proposal for the new scrutiny panel procedure, which it said would "hamper innovation" whilst providing no extra safety nets for patients.
"The current regulatory framework has provided a high level of safety for patients in Europe without delaying them access to life-saving medical technologies," Serge Bernasconi, Eucomed's chief executive, said in a statement.
"Let's not unnecessarily push away Europe's strong innovation and research capabilities to other continents at a time when they are urgently needed."
European Consumer Organisation (BEUC) argued, on the other hand, that the plans fall short of increasing quality and safety standards and said medical device regulations should be beefed up to levels similar to those required for pharmaceuticals.
"It is unacceptable that consumers are afforded different protection levels depending whether they have an artificial heart valve or take medicine for diabetes," said Monique Goyens, head of BEUC.
She noted that if a there is a problem with a drug, patients can stop taking them, but if an implanted device is problematic, patients may face invasive and risky surgery to have it removed.
Among other main changes proposed is an extension of the current legal definition of medical devices to include breast and other aesthetic implants.
Independent assessment agencies will be given greater powers to monitor device manufacturers, including unannounced factory inspections and regular product testing, while EU governments will be obliged to improve their supervision of the agencies.
Better product traceability systems will also be introduced so that people can be alerted more rapidly to safety concerns surrounding a particular device.
The European market for medical devices was estimated at 95 billion euros (123 billion) in 2009.
The legislation must be jointly approved by EU governments and lawmakers, which could take up to two years.
($1 = 0.7715 euros)
(Editing by James Jukwey and Hans-Juergen Peters)
BY:503832
Cannot play media. You do not have the correct version of the flash player. Download the correct version
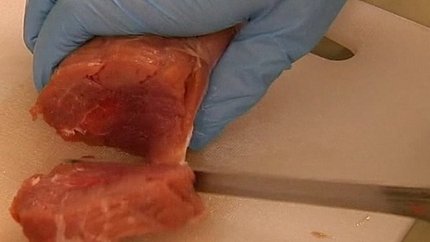
Swedish alert over fake pork meat
Sweden's National Food Agency has issued a warning after as much as 20 tonnes of meat labelled as beef turned out to be coloured pork.
An investigator at the agency, Pontus Elvingson, told the BBC that tests were still being done to identify the dye.
The Swedish firm Heat AB imported the meat from a supplier in Hungary called Filetto. One of the suspect batches originated in Argentina.
Checks show that the dyed meat was first sold in Sweden a year ago.
It is not yet clear if Filetto also exported doctored pork to other countries. The Swedish agency has alerted EU authorities.
The BBC was unable to reach Filetto for comment.
Contamination threat Mr Elvingson said the fake beef had been sold to several Swedish retail outlets, including restaurants. So far about 3.5 tonnes has been removed from sale.
"There is no indication that Heat AB sent any of the meat abroad," he told the BBC by phone. Heat AB was not registered as a food company, he added.
In Sweden, he said, "it's difficult to tell how much [doctored meat] there is and we don't know if it has all been sold".
"The pigmentation of beef is different - this meat is red, but seems not so well dyed in parts, so maybe it was injected with needles," he said.
If needles were used to inject the dye then bacteria could have been transferred from the meat's surface to the interior, increasing the risk of food poisoning, he added.
The agency was tipped off by Swedish wholesaler Svensk Cater, following a complaint from a customer.
Heat AB's Managing Director Ake Hultberg told Reuters news agency that the meat he had tried was good.
"When we received the product, I looked at it, I opened a box and took a sample which I myself fried and looked at it.
"And there was no problem with it but when it later came to Svensk Cater, there were fillets that were not beef but pork," he said, insisting that parts of the delivery were real beef.
 RSS Feed
RSS Feed